Background Acute myeloid leukemia (AML) is a heterogeneous hematologic malignancy characterized by the clonal expansion of malignant progenitor cells and impaired cell differentiation. GL-V9, a nature compound derived from wogonin, has shown superior anti-tumor potential by inhibiting cancer cell growth. Azacitidine (AZA), a DNA hypomethylation agent, has demonstrated therapeutic efficacy against AML. In this study, we investigated the anti-leukemia effect and potential mechanism of AZA combined with GL-V9 in AML cells, aiming to provide evidence for future clinical treatment.
Methods We employed Cell Counting Kit-8 (CCK-8) to assess cell viability, Annexin-V/PI staining followed by flow cytometry analysis to measure apoptosis. CalcuSyn analysis was used to evaluate the synergistic effect. RNA-seq was performed in U937 cells and differentially expressed genes (DEGs) were identified and subjected to KEGG (Kyoto Encyclopedia of Genes and Genomes) analysis. RT-qPCR and Western Blot were used to examine gene expression.
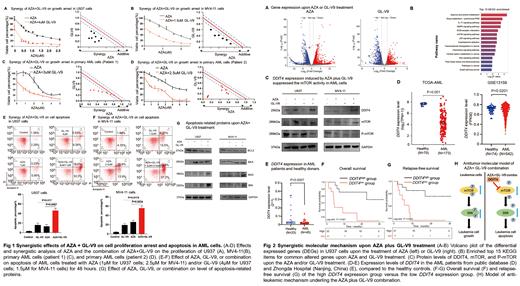
Results Our results demonstrated significant cell proliferation arrest in U937 and MV4-11 cells treated with the combination of AZA and GL-V9, compared to the single drug ( Fig. 1A&B). CalcuSyn analysis showed a strong synergistic effect for the combination ( Fig. 1A&B). Similar results were also found in primary AML cells derived from 2 AML patients [patient 1 exhibited t(6;11) (q27;q23) chromosomal translocation, while Patient 2 presented with secondary AML, transformed from CMML (Chronic Myelomonocytic Leukemia) and featured ASXL1 and SRSF2 mutations] ( Fig. 1C&D). Moreover, AZA plus GL-V9 induced a substantial increase in cell apoptosis in U937 and MV4-11 cells compared to the single drug ( Fig. 1E&F). Consistently, the AZA+GL-V9 combination led to a notable increase in the protein level of Bax, BAD, BIM, and a decrease in BCL2 ( Fig. 1G). These data indicated the combination of AZA with GL-V9 has synergistic anti-leukemia effects in AML. To understand the underlying mechanisms of this synergy, we performed RNA-seq analysis in U937 cells, identifying 1385 and 586 DEGs (|log 2FC|≥2.0, P<0.05) upon AZA or GL-V9 treatment, respectively ( Fig. 2A). The mTOR signaling pathway exhibits prominent enrichment in the KEGG analysis of the overlapping DEGs between AZA and GL-V9 ( Fig. 2B). DDIT4, a negative regulator of mTOR emerged as one of the top DEGs in response to both AZA and GL-V9 treatment. Importantly, the AZA+GL-V9 treatment exhibited a higher expression level of DDIT4 compared to either drug alone and suppressed the phosphorylation of mTOR ( Fig. 2C). Additionally, the DDIT4 expression level was significantly decreased in AML patients, which derived from a public database (TCGA-AML, GSE13159)] ( Fig. 2D) and Zhongda Hospital (Nanjing, China) compared to the healthy controls ( Fig. 2E). To be noticed, high DDIT4 expression was associated with extended overall survival (P=0.021) ( Fig. 2F) and relapse-free survival (P=0.015) ( Fig. 2G), indicating DDIT4 may act as a tumor suppressor in AML. These findings suggested that the combination may exert the anti-leukemia effect by targeting the DDIT4/mTOR signaling pathway, as summarized in Fig. 2H.
Conclusions Our study provides novel evidence of the synergistic effect on cell growth arrest and apoptosis treated with a new nature compound derived from wogonin combined with AZA in AML. Furthermore, we identified the mechanism underlying the synergy through targeting of DDIT4/mTOR signaling. These results offer preliminary support for the potential application of this combination therapy in treating AML patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal